Meet Dr. Charlène Danoux.
Head of QA/QC/RA
Dr. Charlene Danoux brings extensive expertise in Quality and Regulatory Affairs, honed through senior roles at renowned organizations such as AGC Chemicals and BSI. Her deep knowledge spans material science and regulatory compliance, making her an invaluable asset to our team.
With a Ph.D. in Materials for Regenerative Medicine, earned in 2016, Dr. Danoux is dedicated to ensuring that our clients consistently receive the outstanding quality and excellence they have come to trust. Together with our team, she is committed to driving innovation and maintaining the highest standards for our partners.
Tomorrow's Standards, Today
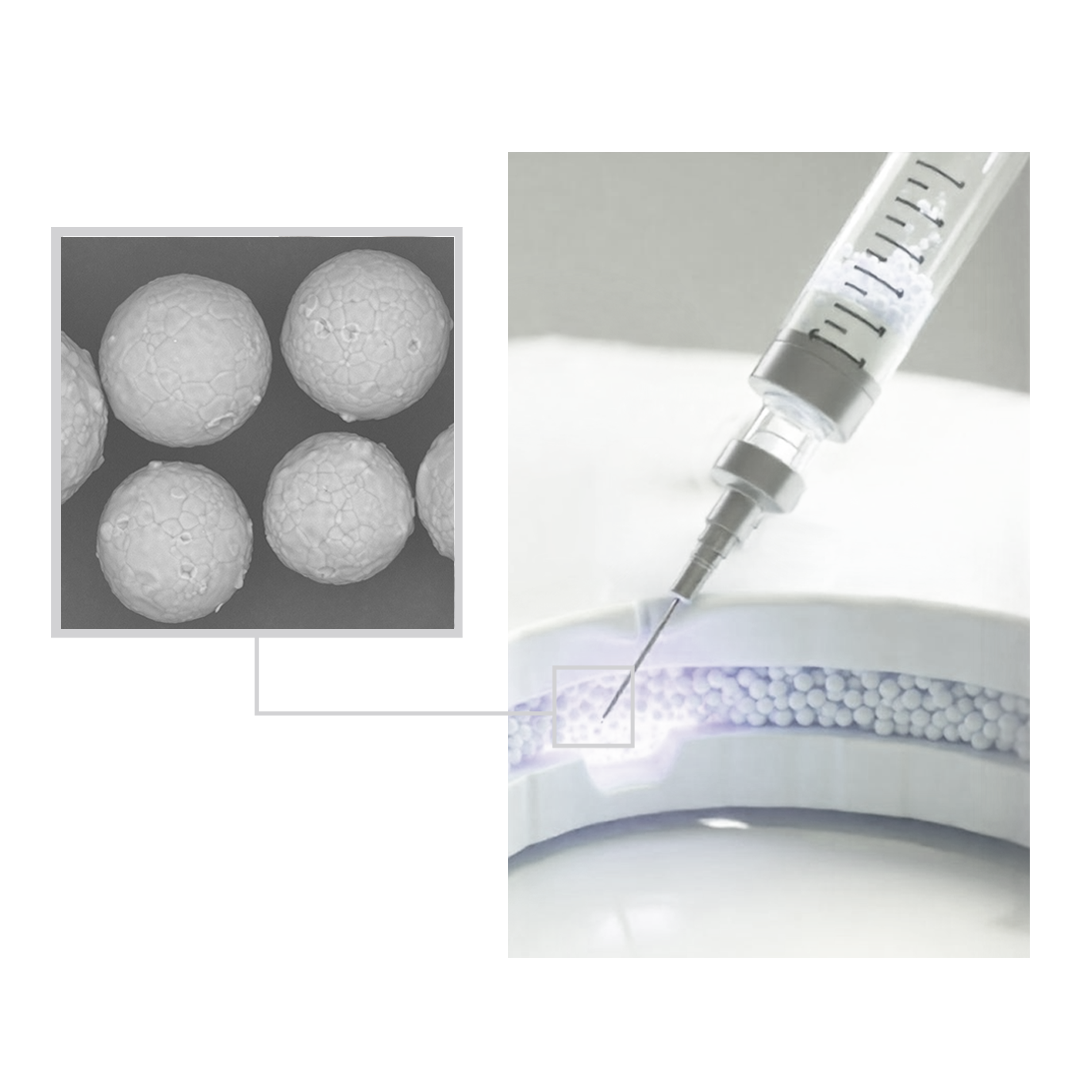
In the dynamic and competitive world of aesthetics, staying ahead demands constant innovation. Calcium Hydroxyapatite (CaHA) is rapidly becoming the material of choice for its outstanding regenerative properties and long-lasting results.
Our dedicated QA/QC/RA team is here to support clients in accelerating the launch of their new CaHA dermal fillers. From the early development stages to full-scale production, we handle all validation and testing processes in-house, ensuring strict adherence to consistent, high-quality standards.
Our in-process controls guarantee compliance with relevant standards (ISO 13485:2016, USP, and Ph. Eur) while also ensuring that we maintain batch-to-batch consistency .Laser diffraction, density measurements and SEM imaging for particle characterization.
Unlike traditional lab-based approaches, we manufacture CaHA using advanced production equipment. This scalable approach safeguards process validation and streamlines production, eliminating common challenges associated with scaling up.
Our approach of using in-house production of the raw materials allows us to control the output to the highest possible degree. All of the in-process and final controls you would expect are available:
-
XRD, FTIR and titration measurements on chemical composition
-
Trace element quantification
-
Laser diffraction, density measurements and SEM imaging for particle characterization
-
S-BET for the characterization of the particle surface
We love challenges and we welcome customer requests that push us to innovate and improve. We have experience in developing inhouse methods when no standards are available. We regularly investigate new techniques and methods to stay at the forefront of our domain.
Development Program
We have successfully guided leading aesthetics companies through the process of obtaining FDA, ANVISA, and EU regulatory approvals for integrating CaHA into their product portfolios. Now, it's your turn. Let’s explore how we can support you in successfully incorporating CaHA into your offerings.
Updates
.jpeg?width=250&name=image%20(2).jpeg)
Calcium Hydroxyapatite (CaHA) fillers provide a unique blend of natural-looking results and long-lasting volume restoration, setting them apart from other dermal fillers. With its mineral-based composition, CaHA not only enhances skin structure...

In the competitive realm of the aesthetic market, staying ahead requires continuous innovation. Companies around the world are increasingly turning to Calcium Hydroxyapatite (CaHA) for its exceptional regenerative properties and enduring results.

The aesthetics industry is evolving, and the spotlight is shifting towards skin regeneration—a revolutionary approach that enhances natural beauty from within. Here's why it's time to embrace this trend
